Instructions For Use
ŌNŌCOR 12F ŌNŌ RETRIEVAL DEVICE
DEVICE DESCRIPTION
The 12F ŌNŌ retrieval device consists of a series of braided loops forming a basket fused to a 7.5F inner diameter catheter. A peel away sheath is preloaded over the ŌNŌ shaft which will assist in compression of the basket for introduction through the hemostasis valve of a 12F or larger vascular sheath.
INDICATIONS FOR USE
The Onocor ŌNŌ Retrieval device is indicated for use in the cardiovascular system to retrieve foreign objects using minimally invasive surgical procedures. Procedures include retrieval of intravascular foreign objects such as coils, balloons, catheters, guidewires, and/or filters within the cardiovascular system. This device is not intended for use in the coronary arteries or neurovasculature.
CONTRAINDICATIONS
This device is not intended for the removal of foreign objects entrapped by tissue growth.
WARNINGS
This product contains nitinol, a nickel-titanium alloy. Allergic reactions to nickel should be considered.
CAUTION: US federal law restricts this device to sale by or on the order of a physician (or properly licensed practitioner).
PRECAUTIONS
This is intended for use by physicians trained and experienced in diagnostic and interventional techniques. Standard techniques for placement of vascular access sheaths, angiographic catheters and guidewires should be employed.
Manipulation of the product requires fluoroscopic control.
Excessive forces should not be used to manipulate or retrieve foreign objects.
Do not attempt to shape the catheter tip or basket, as doing so may damage the device. Damage may include, but not limited to, a compromise of the inner lumen diameter.
The ŌNŌ is not indicated for pressure injection. The ŌNŌ allows for hand injections performed with a 10 mm or larger syringe.
POTENTIAL ADVERSE EVENTS
Potential adverse procedural events related to foreign body manipulation and retrieval in the vasculature can include, but are not limited to:
Embolization
Pulmonary embolism
Vessel perforation
Stroke
Myocardial infarction
Device entrapment
HOW SUPPLIED
Supplied sterilized by ethylene oxide gas in peel-open packages. Intended for one-time use. Sterile if package is unopened and undamaged. Do not use the product if there is doubt as to whether the product is sterile. Store in a dark, dry, cool place. Avoid extended exposure to light. Upon removal from package, inspect the product to ensure no damage has occurred.
After use, dispose of product and packaging in accordance with hospital, administrative and/or local government policy.
INSTRUCTIONS FOR USE
Remove the ŌNŌ device from packaging
Advance the peel away sheath over the braided basket.
Flush the peel away sheath lumen with heparinized saline.
Flush the catheter lumen with heparinized saline.
Insert the tip of the peel away sheath into the retrieval sheath just past the hemostasis valve (minimum diameter 12Fr; maximum length 75cm). Advance the basket into the lumen of the retrieval sheath.
If desired, remove the peel away sheath from the shaft of the ŌNŌ.
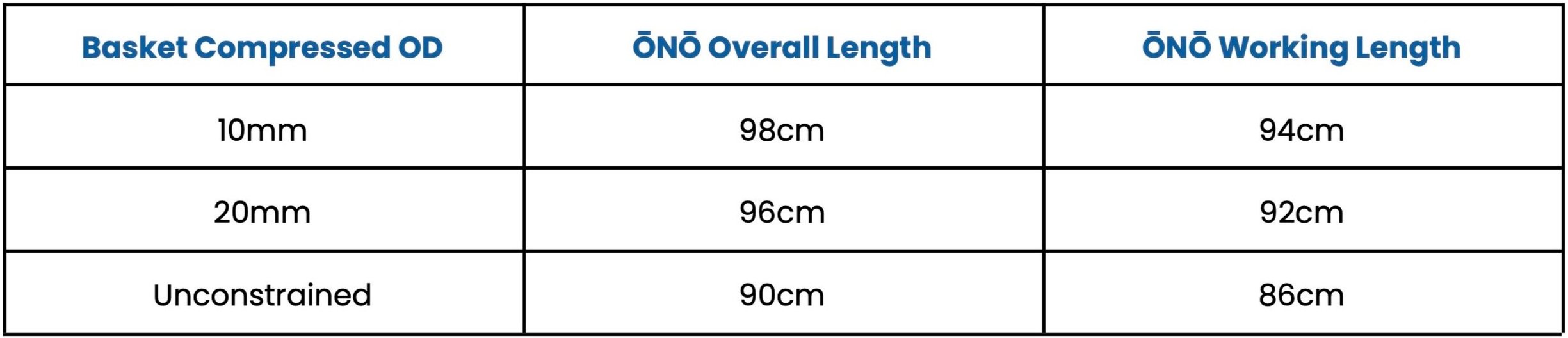
Under fluoroscopic guidance advance the ŌNŌ through the retrieval sheath and deploy the basket adjacent to the foreign body to be retrieved. Note that the basket shortens upon deployment affecting the overall and working lengths of the system.
Advance a snare or other manipulation device through the lumen of the ŌNŌ (maximum diameter 7Fr; minimum length 100cm).
Grasp the targeted foreign body with the snare and retract it into the basket. Note: if resistance is felt, re-assess the position of the foreign body and snare before proceeding.
Stabilize the retrieval sheath and withdraw the snare and foreign body into the retrieval sheath simultaneously. Note: if resistance is felt, re-assess the position of the foreign body and snare before proceeding.
Remove the foreign object from the retrieval sheath or remove the foreign body / retrieval sheath simultaneously. Note: if resistance is felt, re-assess the position of the foreign body and snare before proceeding.
After retrieval, hospital standard of care should be followed for removing the sheath and providing hemostasis to prevent bleeding at the vascular access site. Dispose of all used devices in accordance with hospital policy for bio hazardous materials.
IDENTIFICATION OF SYMBOLS USED ON PRODUT LABELING